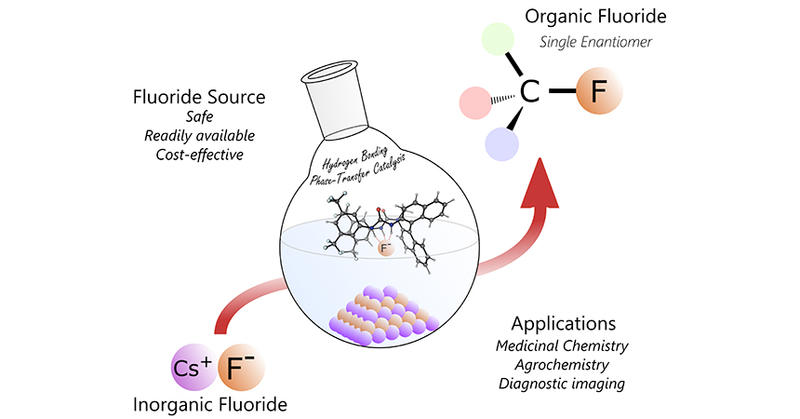
The Gouverneur laboratory has focused for many years on development of innovative (catalytic) reactions for the fluorination and polyfluoroalkylation of alkenes with regio-, diastereo- and enantiocontrol (ACIE 2008, ACIE 2011 (x2), JACS 2013). This body of work was extended to the site-selective late stage difluoroalkylation of biologically relevant dehydroalanine-containing proteins under photoredox catalysis (Nature 2020). The enhancement of our fundamental understanding of alkali metal fluoride reactivity is also central to her research, with a body of work that led to the invention of Hydrogen Bonding Phase Transfer Catalysis (HBPTC), a novel concept opening new opportunities in organic chemistry (Science 2018). HBPTC consists of a chiral non-racemic hydrogen bond donor (bis)urea catalyst capable of transporting solid nucleophile reagents as simple as inorganic salts into solution, and therefore enabling asymmetric carbon-nucleophile bond formation. HBPTC was successfully applied to alkali metal fluoride in combination with episulfonium, aziridinium or azetidinium electrophiles affording enantioenriched fluorinated building blocks for drug discovery (JACS 2019, JACS 2020). This work led to in-depth understanding of the influence of hydrogen bonding on fluoride reactivity and its application to asymmetric catalytic nucleophilic fluorination (JACS 2020), and new ways to reroute regioselectivity for the fluorintion of unsymmetrical aziridinium salts (JACS 2023). The impact of this work is far reaching as the concept of hydrogen bonding phase transfer catalysis has applications beyond fluoride, as recently demonstrated with enantioselective azidation with NaN3 (JACS 2022). The new N-alkylated (bis)urea catalyst employed in HBPTC is commercially available and a protocol for its large-scale synthesis is available (Nat. Prot. 2021).